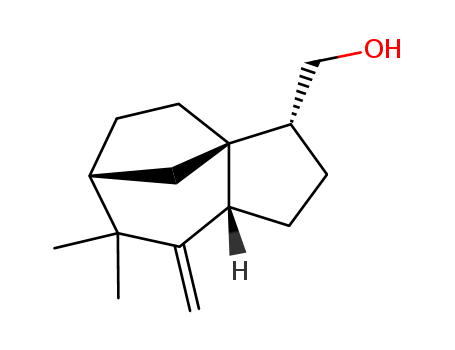
Chemical Property of Khusimol
Edit
Chemical Property:
- Vapor Pressure:4.4E-05mmHg at 25°C
- Boiling Point:313.2°Cat760mmHg
- PKA:15.00±0.10(Predicted)
- Flash Point:119.7°C
- PSA:20.23000
- Density:1.02g/cm3
- LogP:3.38740
- XLogP3:3.7
- Hydrogen Bond Donor Count:1
- Hydrogen Bond Acceptor Count:1
- Rotatable Bond Count:1
- Exact Mass:220.182715385
- Heavy Atom Count:16
- Complexity:330
- Purity/Quality:
-
99% *data from raw suppliers
Khusenol *data from reagent suppliers
Safty Information:
- Pictogram(s):
- Hazard Codes:
- MSDS Files:
-
Useful:
- Canonical SMILES:CC1(C2CCC3(C2)C(CCC3C1=C)CO)C
- Isomeric SMILES:CC1([C@@H]2CC[C@]3(C2)[C@H](CC[C@@H]3C1=C)CO)C
-
Uses
Khusenol, is a component of Vetiver essential oil which is a highly esteemed basic ingredient of modern perfumery, hvaing a woody, earthy scent.