- Chemical Name:Iodomethane
- CAS No.:74-88-4
- Deprecated CAS:147937-07-3,1519044-66-6,1173018-72-8
- Molecular Formula:CH3I
- Molecular Weight:141.939
- Hs Code.:2903399030
- European Community (EC) Number:200-819-5
- ICSC Number:0509
- NSC Number:9366
- UN Number:2644
- UNII:DAT010ZJSR
- DSSTox Substance ID:DTXSID0024187
- Nikkaji Number:J1.063.962J,J1.245.462G,J2.382E
- Wikipedia:Iodomethane
- Wikidata:Q421729,Q83037351
- Metabolomics Workbench ID:56218
- ChEMBL ID:CHEMBL115849
- Mol file:74-88-4.mol
Synonyms:iodomethane;iodomethane-D3;methyl iodide;methyl iodide, 11C-labeled;methyl iodide, 131I-labeled;methyl iodide, 132I-labeled;methyl iodide, 13C-labeled;methyl iodide, 14C-labeled;methyl iodide, 2H-labeled

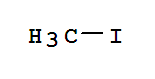
 T,
T,  F,
F,  Xn
Xn
 F:Flammable;
F:Flammable;


