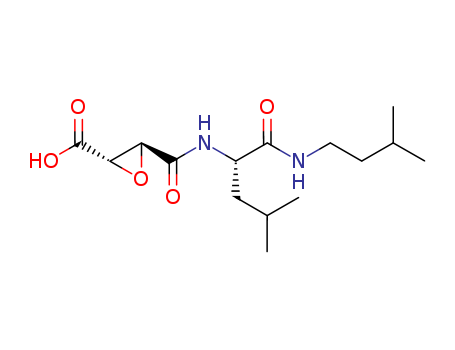
- Chemical Name:Oxiranecarboxylic acid, [2S-[2alpha,3beta(R*)]]-
- CAS No.:76684-89-4
- Molecular Formula:C15H26 N2 O5
- Molecular Weight:314.382
- Hs Code.:29241990
- NSC Number:694279
- DSSTox Substance ID:DTXSID10997997
- ChEMBL ID:CHEMBL1973415
- Mol file:76684-89-4.mol
Synonyms:C15H26N2O5;C15-H26-N2-O5;SCHEMBL6041204;CHEMBL1973415;DTXSID10997997;NSC694279;NSC-694279;NCI60_033763;A903413;Oxiranecarboxylic acid, [2S-[2.alpha.,3.beta.(R*)]]-;(2S,3S)-3-(((S)-1-(isopentylaMino)-4-Methyl-1-oxopentan-2-yl)carbaMoyl)oxirane-2-carboxylic acid;Loxistatin acid;3-[Hydroxy({1-hydroxy-4-methyl-1-[(3-methylbutyl)imino]pentan-2-yl}imino)methyl]oxirane-2-carboxylic acid