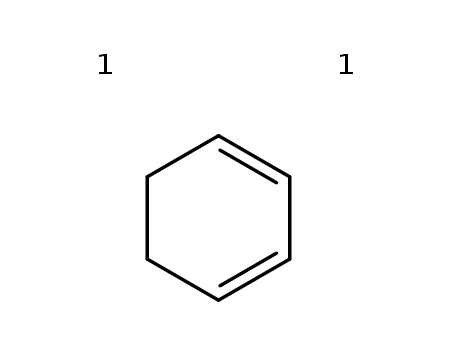
- Chemical Name:1,3-Cyclohexadiene
- CAS No.:592-57-4
- Deprecated CAS:33004-09-0
- Molecular Formula:C6H8
- Molecular Weight:80.1295
- Hs Code.:29021990
- European Community (EC) Number:249-853-2,209-764-1
- ICSC Number:0762
- UN Number:1993
- UNII:JV5W0EG5BP
- DSSTox Substance ID:DTXSID30862259
- Nikkaji Number:J1.855.158F,J1.855.159D,J94.915I
- Wikipedia:Cyclohexa-1,3-diene
- Wikidata:Q387320
- Metabolomics Workbench ID:55637
- Mol file:592-57-4.mol
Synonyms:1,3-cyclohexadiene