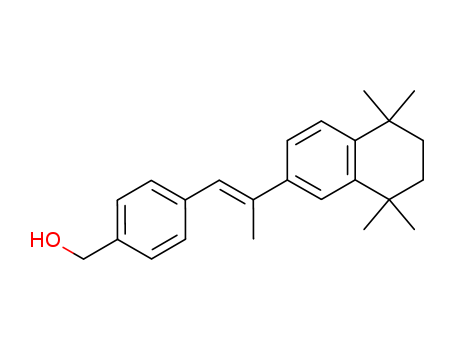
- Chemical Name:Arotinoic methanol
- CAS No.:71441-30-0
- Molecular Formula:C24H30 O
- Molecular Weight:334.502
- Hs Code.:
- Nikkaji Number:J325.996J
- Mol file:71441-30-0.mol
Synonyms:4 (2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propen-1-yl)phenylmethanol;arotinoic methanol;arotinoid Ro 13-8320;Ro 13-8320;Ro-13-8320