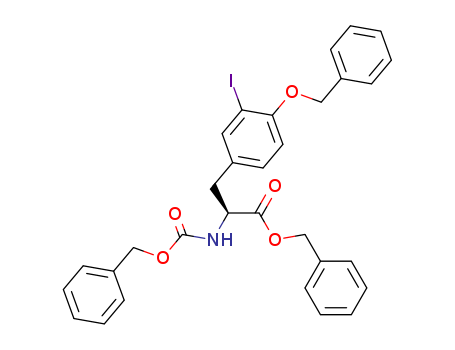
- Chemical Name:3-Iodo-N-[(benzyloxy)carbonyl]-O-benzyl-L-tyrosine Benzyl Ester
- CAS No.:600737-79-9
- Molecular Formula:C31H28INO5
- Molecular Weight:621.472
- Hs Code.:
- Mol file:600737-79-9.mol
Synonyms:3-Iodo-N-[(benzyloxy)carbonyl]-O-benzyl-L-tyrosine Benzyl Ester;