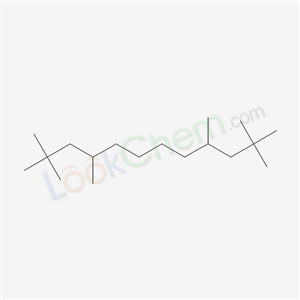
- Chemical Name:2,2,4,9,11,11-Hexamethyldodecane
- CAS No.:6304-50-3
- Molecular Formula:C18H38
- Molecular Weight:254.4943
- Hs Code.:2901100000
- NSC Number:42965
- DSSTox Substance ID:DTXSID00285830
- Nikkaji Number:J103.569J
- Mol file:6304-50-3.mol
Synonyms:2,2,4,9,11,11-Hexamethyldodecane;6304-50-3;Dodecane, 2,2,4,9,11,11-hexamethyl-;NSC42965;DTXSID00285830;2,4,9,11,11-Hexamethyldodecane;NSC-42965;Dodecane,2,4,9,11,11-hexamethyl-;2,2,4,9,11,11-hexamethyl-dodecane