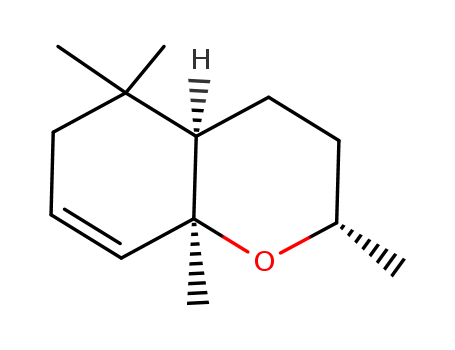
- Chemical Name:(-)-dihydroedulan II
- CAS No.:41678-32-4
- Molecular Formula:C13H22O
- Molecular Weight:194.317
- Hs Code.:
- DSSTox Substance ID:DTXSID601316713
- Nikkaji Number:J238.383G
- Wikidata:Q27159863
- Mol file:41678-32-4.mol
Synonyms:(-)-dihydroedulan II;Dihydroedulan II;SCHEMBL20873434;CHEBI:87716;DTXSID601316713;Q27159863;(2S,4aR,8aS)-2,5,5,8a-tetramethyl-3,4,4a,6-tetrahydro-2H-chromene;3,4,4abeta,5,6,8a-Hexahydro-2beta,5,5,8abeta-tetramethyl-2H-1-benzopyran;(2S,4aR,8aS)-2,5,5,8a-tetramethyl-3,4,4a,5,6,8a-hexahydro-2H-1-benzopyran