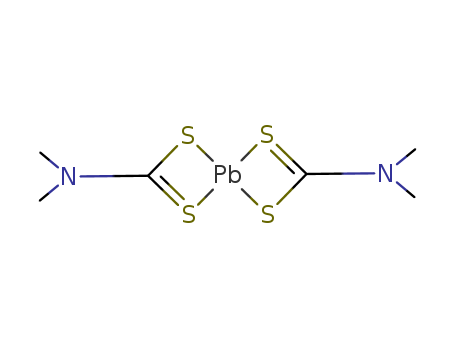
- Chemical Name:Lead dimethyldithiocarbamate
- CAS No.:19010-66-3
- Molecular Formula:C6H12N2PbS4
- Molecular Weight:447.639
- Hs Code.:2930909090
- European Community (EC) Number:242-748-2
- ICSC Number:1545
- Wikidata:Q27292124
- Mol file:19010-66-3.mol
Synonyms:Lead dimethyldithiocarbamate;bis(dimethylcarbamothioylsulfanyl)lead;19010-66-3;Lead bis(dimethyldithiocarbamate);Lead dimethyl dithiocarbamate;CCRIS 359;HSDB 2886;Lead Dimethyldithiocarbamate -Arc;NCI-C02891;Lead, bis(dimethyldithiocarbamato)-;EINECS 242-748-2;LS-960;Dimethyldithiocarbamic acid, lead salt;Bis(dimethylcarbamodithioato-S,S')lead;Carbamic acid, dimethyldithio-,lead salt;Lead, bis(dimethylcarbamodithioato-S,S')-, (T-4)-;Q27292124;Lead, bis(dimethylcarbamodithioato-S,S')-, (beta-4)-;Lead, bis(dimethylcarbamodithioato-kappaS,kappaS')-, (T-4)-;N,N,6-trimethyl-1,5-dithioxo-2,4-dithia-6-aza-3-plumbaheptan-1-amine