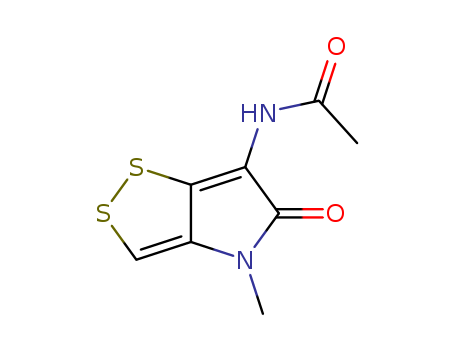
- Chemical Name:Thiolutin
- CAS No.:87-11-6
- Molecular Formula:C8H8N2O2S2
- Molecular Weight:228.296
- Hs Code.:29419090
- European Community (EC) Number:635-840-2
- NSC Number:3927
- UNII:02C005Q20B
- DSSTox Substance ID:DTXSID0040624
- Nikkaji Number:J4.271D
- Wikipedia:Thiolutin
- Wikidata:Q7784674
- NCI Thesaurus Code:C64175
- Metabolomics Workbench ID:46432
- ChEMBL ID:CHEMBL507026
- Mol file:87-11-6.mol
Synonyms:acetopyrrothine;thiolutin