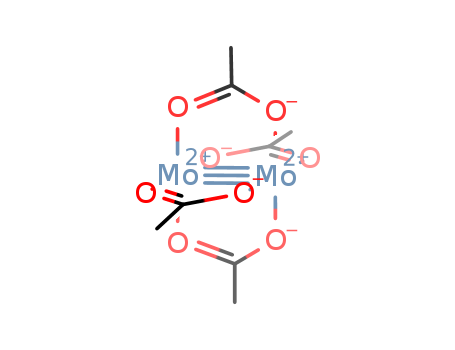
- Chemical Name:MOLYBDENUM(II) ACETATE DIMER
- CAS No.:14221-06-8
- Molecular Formula:C8H12Mo2O8
- Molecular Weight:428.058
- Hs Code.:2915900090
- European Community (EC) Number:238-089-5
- Wikipedia:Molybdenum(II)_acetate
- Mol file:14221-06-8.mol
Synonyms:Molybdenum,tetrakis(acetato)di- (7CI); Molybdenum, tetrakis(m-acetato)di-, (Mo-Mo) (8CI); Molybdenum, tetrakis[m-(acetato-O:O')]di-, (Mo-Mo);Dimolybdenum tetraacetate; Tetraacetatodimolybdenum;Tetrakis(acetato)dimolybdenum; Tetrakis(acetato)molybdenum; Tetrakis(m-acetato)dimolybdenum