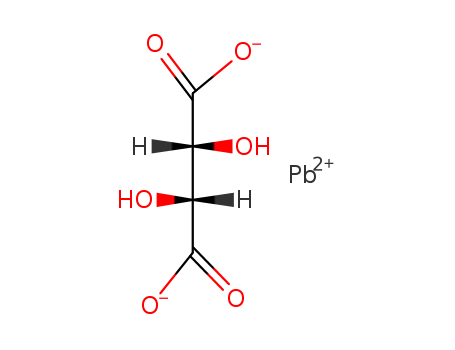
- Chemical Name:LEAD(II) TARTRATE
- CAS No.:815-84-9
- Molecular Formula:C4H6 O6 . Pb
- Molecular Weight:355.272
- Hs Code.:
- Mol file:815-84-9.mol
Synonyms:Butanedioicacid, 2,3-dihydroxy- [R-(R*,R*)]-, lead(2+) salt (1:1); Lead tartrate(6CI,7CI); Tartaric acid, lead(2+) salt (1:1) (8CI); NSC 1914