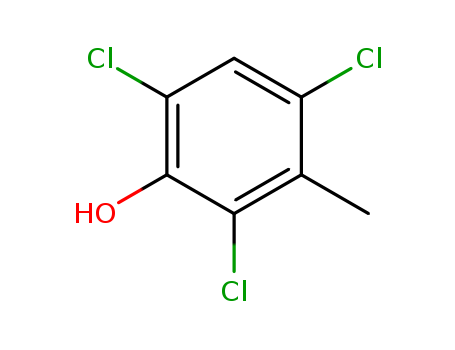
- Chemical Name:2,4,6-Trichloro-m-cresol
- CAS No.:551-76-8
- Molecular Formula:C7H5 Cl3 O
- Molecular Weight:211.475
- Hs Code.:2908199090
- UNII:D0H0QF5KL9
- DSSTox Substance ID:DTXSID30203623
- Nikkaji Number:J28.694J
- Wikidata:Q27275937
- Mol file:551-76-8.mol
Synonyms:2,4,6-Trichloro-m-cresol;2,4,6-TRICHLORO-3-METHYLPHENOL;551-76-8;Phenol, 2,4,6-trichloro-3-methyl-;UNII-D0H0QF5KL9;D0H0QF5KL9;2,4,6-Trichlor-m-kresol;SCHEMBL10771021;DTXSID30203623;2,4,6-trichloro-3-hydroxytoluene;M-CRESOL, 2,4,6-TRICHLORO-;2,4,6-TRICHLORO-M-CRESOL [MI];Q27275937