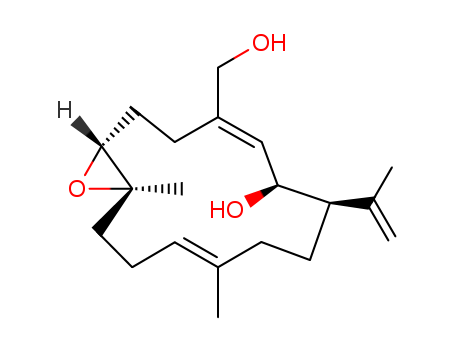
- Chemical Name:Asperdiol
- CAS No.:64180-67-2
- Molecular Formula:C20H32O3
- Molecular Weight:320.472
- Hs Code.:
- NSC Number:279559
- DSSTox Substance ID:DTXSID00418489
- ChEMBL ID:CHEMBL1989490
- Mol file:64180-67-2.mol
Synonyms:ASPERDIOL;64180-67-2;NSC279559;CHEMBL1989490;DTXSID00418489;15-Oxabicyclo[12.1.0]pentadeca-4,10-diene-4-methanol, 6-hydroxy-10,14-dimethyl-7-(1-methylethenyl)-, [1R-(1R*,4E,6S*,7R*,10E,14R*)]-;NSC-279559