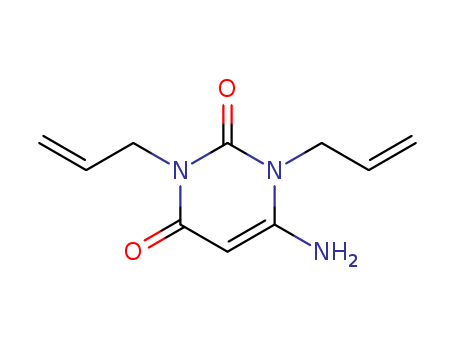
- Chemical Name:1,3-Diallyl-6-aminouracil monohydrate
- CAS No.:4852-19-1
- Molecular Formula:C10H13 N3 O2
- Molecular Weight:207.232
- Hs Code.:
- European Community (EC) Number:225-444-4
- DSSTox Substance ID:DTXSID60964078
- Nikkaji Number:J236.622C
- Wikidata:Q82945994
- Mol file:4852-19-1.mol
Synonyms:1,3-Diallyl-6-aminouracil;1,3-Diallyl-6-aminouracil monohydrate;4852-19-1;EINECS 225-444-4;SCHEMBL476495;DTXSID60964078;1,3-Diallyl-6-amino-2,4(1H,3H)-pyrimidinedione #;2,4(1H,3H)-Pyrimidinedione, 6-amino-1,3-di-2-propenyl-;6-Amino-1,3-di(prop-2-en-1-yl)pyrimidine-2,4(1H,3H)-dione