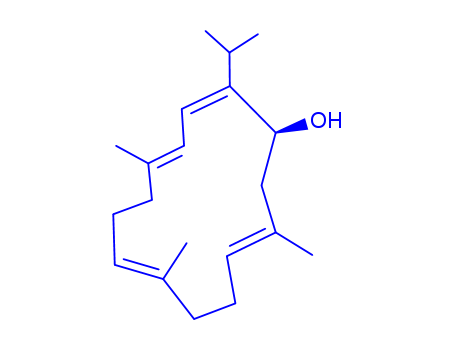
- Chemical Name:Sarcophytol A
- CAS No.:72629-69-7
- Deprecated CAS:88979-97-9
- Molecular Formula:C20H32O
- Molecular Weight:288.473
- Hs Code.:2906199090
- UNII:240025685W
- DSSTox Substance ID:DTXSID5021257
- Wikidata:Q27253783
- Metabolomics Workbench ID:143725
- ChEMBL ID:CHEMBL462849
- Mol file:72629-69-7.mol
Synonyms:(1S,2Z,4E,8E,12E)-2-Isopropyl-5,9,13-trimethyl-2,4,8,12-cyclotetradecatetraen-1-ol;sarcophytol A