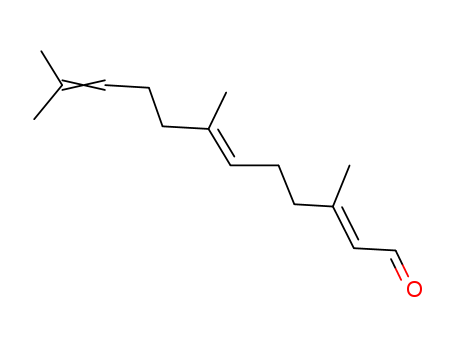
- Chemical Name:Farnesal
- CAS No.:502-67-0
- Deprecated CAS:807656-93-5
- Molecular Formula:C15H24 O
- Molecular Weight:220.355
- Hs Code.:
- European Community (EC) Number:242-957-9
- UNII:G4E58106EW
- ChEMBL ID:CHEMBL3120646
- DSSTox Substance ID:DTXSID60880981
- Metabolomics Workbench ID:28207
- Nikkaji Number:J1.852.519D,J14.249B,J672.102H,J672.103F,J102.721B
- Wikidata:Q27098285
- Mol file:502-67-0.mol
Synonyms:3,7,11-trimethyldodeca-2,6,10-trienal;farnesal