10.1016/0040-4020(95)00378-L
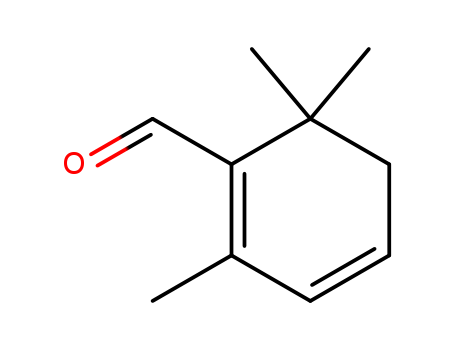
This research aims to develop a concise and efficient synthetic route for the A-ring building block of Taxol, a diterpene with significant antitumor activity but limited natural availability. The study presents a seven-step synthesis starting from an inexpensive mixture of E- and Z-citrals, which are converted to safranal through a series of reactions including cyclization, bromination, and dehydrobromination. Safranal is described as a key intermediate in the synthesis of the A-ring building block for Taxol. It is the major constituent of saffron oil and can be prepared from the inexpensive citral in a few steps. Safranal is used as a starting material to introduce the necessary functional groups and stereochemistry required for the A-ring of Taxol. The researchers reasoned that reduction of the aldehyde function of safranal to an allylic alcohol, followed by protection as the pivalate, would lead to the conjugated allylic pivalate, which could then be further transformed into the desired ketone through allylic oxidation and 1,4-reduction. The authors conclude that their method is advantageous due to its brevity and simplicity, making it a viable alternative for synthesizing the A-ring building block, especially for the development of simpler Taxol analogs with potentially better solubility and therapeutic properties.


 Xi
Xi

