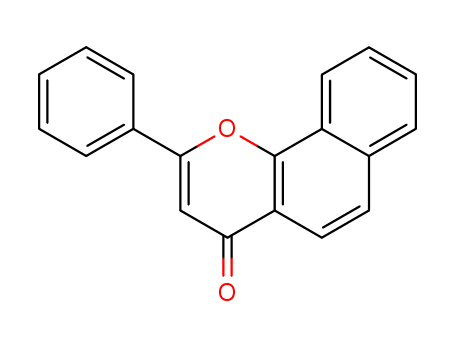
- Chemical Name:alpha-Naphthoflavone
- CAS No.:604-59-1
- Molecular Formula:C19H12O2
- Molecular Weight:272.303
- Hs Code.:29143990
- European Community (EC) Number:210-071-1
- NSC Number:407011
- UNII:FML65D8PY5
- DSSTox Substance ID:DTXSID2040650
- Nikkaji Number:J46.330B
- Wikipedia:Alpha-Naphthoflavone
- Wikidata:Q4734915
- Pharos Ligand ID:CL4Q45WU3SB6
- Metabolomics Workbench ID:74448
- ChEMBL ID:CHEMBL283196
- Mol file:604-59-1.mol
Synonyms:7,8-benzoflavone;alpha-naphthoflavone