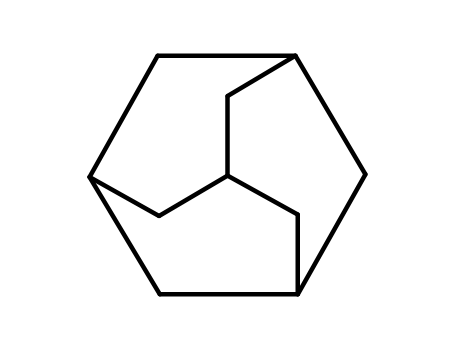
- Chemical Name:Adamantane
- CAS No.:281-23-2
- Molecular Formula:C10H16
- Molecular Weight:136.237
- Hs Code.:29021990
- European Community (EC) Number:206-001-4
- NSC Number:527913
- UNII:PJY633525U
- DSSTox Substance ID:DTXSID5022017
- Nikkaji Number:J45.672A
- Wikipedia:Adamantane
- Wikidata:Q351461,Q83043490,Q83046187
- Metabolomics Workbench ID:52251
- ChEMBL ID:CHEMBL1230831
- Mol file:281-23-2.mol
Synonyms:Adamantane;Diamantane


 Xi
Xi


