10.1039/b801889h
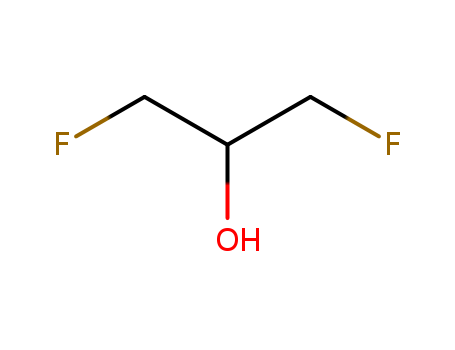
The study focuses on the activation of the carbon-fluorine (C–F) bond in 1,3-difluoro-2-propanol by the nucleophilic attack of the {Pt2S2} core, a complex with significant nucleophilic properties. The reaction proceeds via an SN2 mechanism, where the sulfur atoms in the [Pt2(dppp)2(μ-S)2] complex act as nucleophiles, facilitated by the hydrogen bond from the alcohol group of the organic substrate, which is crucial for the departure of the fluoride anion. The study demonstrates that the presence of an OH group in the substrate plays a key role in the C–F bond activation, and the reaction leads to the formation of a new complex, [Pt2(dppp)2(μ-S)(μ-SCH2CH(OH)CH2F]F. The research also includes theoretical calculations using DFT methods to support the proposed mechanism and to understand the role of the OH group in the reaction. This work not only contributes to the understanding of C–F bond activation but also provides insights into the role of non-covalent interactions, such as hydrogen bonding, in facilitating chemical transformations.