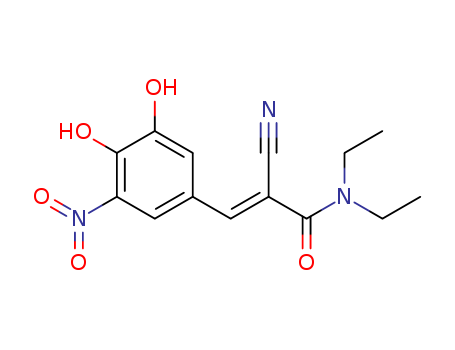
- Chemical Name:Entacapone
- CAS No.:130929-57-6
- Molecular Formula:C14H15N3O5
- Molecular Weight:305.29
- Hs Code.:2926900005
- Mol file:130929-57-6.mol
Synonyms:Entacom;OR 611;2-Propenamide,2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-, (E)-;(E)-Entacapone;Comtan;


 Xn,
Xn, T,
T, F
F

