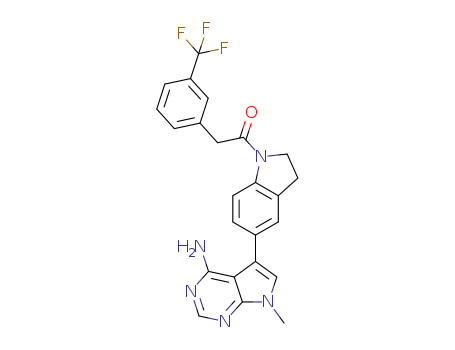
- Chemical Name:1-(5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)indolin-1-yl)-2-(3-(trifluoromethyl)phenyl)ethanone
- CAS No.:1337531-36-8
- Molecular Formula:C24H20F3N5O
- Molecular Weight:451.451
- Hs Code.:
- UNII:DPP2K6EFW8
- Nikkaji Number:J3.338.975B
- Wikipedia:GSK2606414
- Wikidata:Q15409439
- Pharos Ligand ID:2GVXHHLL76MP
- ChEMBL ID:CHEMBL2171124
- Mol file:1337531-36-8.mol
Synonyms:7-methyl-5-(1-((3-(trifluoromethyl)phenyl)acetyl)-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo(2,3-d)pyrimidin-4-amine;GSK2606414