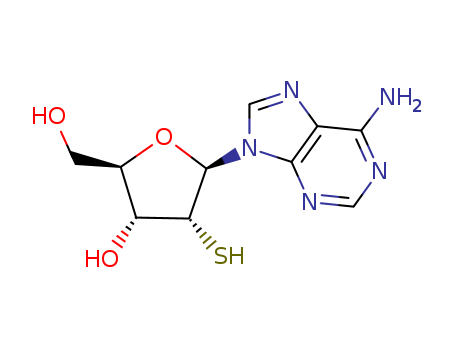
- Chemical Name:2'-Thioadenosine
- CAS No.:136904-69-3
- Molecular Formula:C10H13N5O3S
- Molecular Weight:283.311
- Hs Code.:
- DSSTox Substance ID:DTXSID20435431
- Wikidata:Q82250360
- ChEMBL ID:CHEMBL14326
- Mol file:136904-69-3.mol
Synonyms:2'-Thioadenosine;136904-69-3;CHEMBL14326;(2R,3R,4R,5R)-5-(6-aminopurin-9-yl)-2-(hydroxymethyl)-4-sulfanyloxolan-3-ol;thioadenosine;2/'-Thioadenosine;SCHEMBL2699533;DTXSID20435431;60239-18-1 (non-salt);BDBM50057300;PD138045;J-006937;(2R,3R,4R,5R)-5-(6-Amino-purin-9-yl)-2-hydroxymethyl-4-mercapto-tetrahydro-furan-3-ol