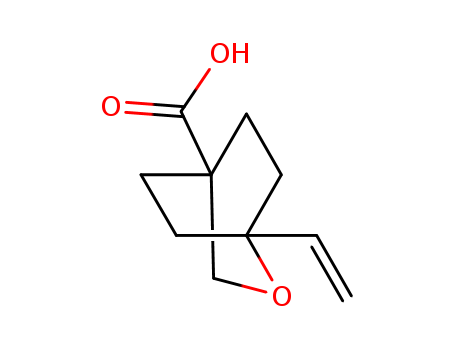
- Chemical Name:1-Ethenyl-2-oxabicyclo[2.2.2]octane-4-carboxylic acid
- CAS No.:340023-04-3
- Molecular Formula:C10H14O3
- Molecular Weight:182.219
- Hs Code.:
- DSSTox Substance ID:DTXSID801214545
- Mol file:340023-04-3.mol
Synonyms:340023-04-3;1-ethenyl-2-oxabicyclo[2.2.2]octane-4-carboxylic acid;1-Vinyl-2-oxabicyclo[2.2.2]octane-4-carboxylic acid;MFCD20658989;SCHEMBL1566353;DTXSID801214545;AKOS025289980;AB91805;AS-51507;SY233987;CS-0058907;P12820;1-Vinyl-2-oxabicyclo[2.2.2]octane-4-carboxylicacid