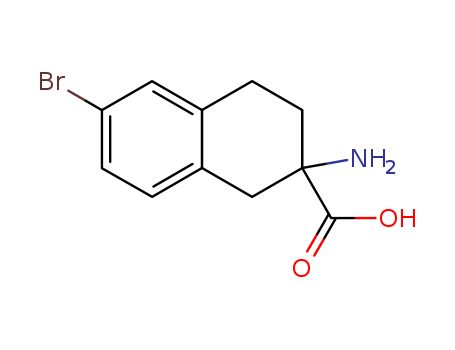
- Chemical Name:2-AMINO-1,2,3,4-TETRAHYDRO-6-BROMO-2-NAPHTHALENE CARBOXYLIC ACID
- CAS No.:659736-91-1
- Molecular Formula:C11H12BrNO2
- Molecular Weight:270.126
- Hs Code.:2922498590
- Mol file:659736-91-1.mol
Synonyms:2-AMINO-1,2,3,4-TETRAHYDRO-6-BROMO-2-NAPHTHALENE CARBOXYLIC ACID;2-Amino-1,2,3,4tetrahydro-6-bromo-2-naphtalene carboxylic acid, 90 %;2-Amino-1,2,3,4-tetrahydro-6-bromo-2-naphtalene carboxylic acid