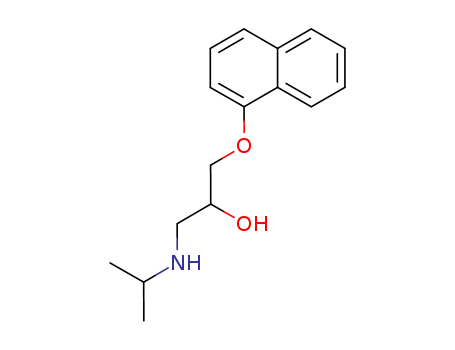
- Chemical Name:()-1-(isopropylamino)-3-(naphthyloxy)propan-2-ol
- CAS No.:13013-17-7
- Molecular Formula:C16H21NO2
- Molecular Weight:259.348
- Hs Code.:
- Mol file:13013-17-7.mol
Synonyms:(±)-1-(isopropylamino)-3-(naphthyloxy)propan-2-ol;dl-Propanolol;rac-(2R*)-1-Isopropylamino-3-(1-naphthalenyloxy)-2-propanol;rac-Propranolol;(1)-1-(Isopropylamino)-3-(naphthyloxy)propan-2-ol;Einecs 235-867-6