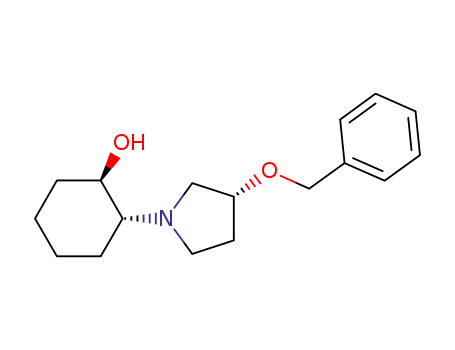
- Chemical Name:2-(3-Benzyloxy-pyrrolidin-1-yl)-cyclohexanol
- CAS No.:900513-88-4
- Molecular Formula:C17H25NO2
- Molecular Weight:275.391
- Hs Code.:
- Mol file:900513-88-4.mol
Synonyms:2-(3-Benzyloxy-pyrrolidin-1-yl)-cyclohexanol;(1R,2R)-2-((R)-3-(benzyloxy)pyrrolidin-1-yl)cyclohexanol