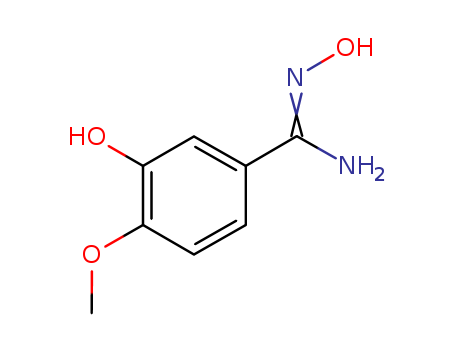
- Chemical Name:N',3-Dihydroxy-4-methoxybenzenecarboximidamide
- CAS No.:352330-51-9
- Molecular Formula:C8H10N2O3
- Molecular Weight:182.1766
- Hs Code.:
- European Community (EC) Number:681-990-7
- ChEMBL ID:CHEMBL1506999
- Mol file:352330-51-9.mol
Synonyms:N,3-Dihydroxy-4-methoxybenzimidamide;352330-51-9;SMR000207723;N',3-Dihydroxy-4-methoxybenzenecarboximidamide;MLS000585942;CHEMBL1506999;HFPJMJWKZLRGNZ-UHFFFAOYSA-N;HMS1375J09;HMS2581B19;AKOS001748428;Benzamidine, 3,N-dihydroxy-4-methoxy-;N',3-Dihydroxy-4-methoxybenzenecarboximidamide #;(Z)-N'3-DIHYDROXY-4-METHOXYBENZENE-1-CARBOXIMIDAMIDE