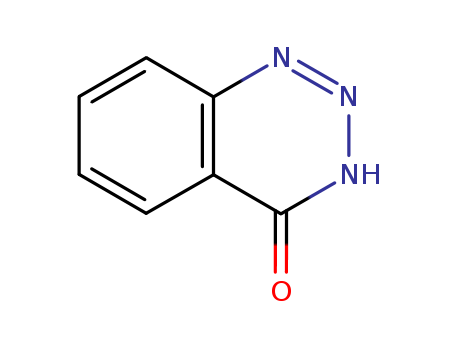
- Chemical Name:1,2,3-Benzotriazin-4(3H)-one
- CAS No.:90-16-4
- Molecular Formula:C7H5N3O
- Molecular Weight:147.136
- Hs Code.:2933698090
- Mol file:90-16-4.mol
Synonyms:3,4-Dihydro-4-oxo-1,2,3-benzotriazine;4-Ketobenz-1,2,3-triazine;Benzazimide;Benzazimidone;NSC 13563;NSC 20121;1,2,3-Benzotriazin-4(1H)-one(7CI,9CI);


 Xi
Xi

