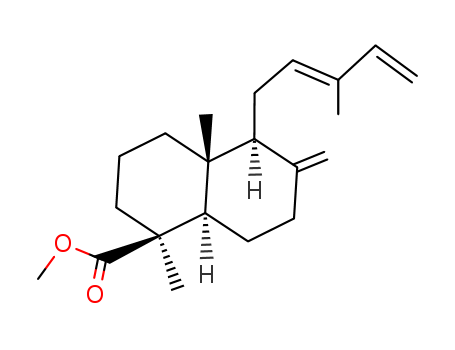
- Chemical Name:Methyl trans-communate
- CAS No.:15798-13-7
- Molecular Formula:C21H32O2
- Molecular Weight:316.484
- Hs Code.:
- UNII:P8PD6BRA3K
- Nikkaji Number:J129.776G,J139.854G,J108.427E
- Wikidata:Q104375917
- Mol file:15798-13-7.mol
Synonyms:Methyl trans-communate;Methyl communate, (E)-;P8PD6BRA3K;15798-13-7;trans-Communic acid methyl ester;UNII-P8PD6BRA3K;Labda-8(20),12,14-trien-19-oic acid, methyl ester, (E)-;Methyl (E)-8(20),12,14-labdatrien-19-oate;Methyl cis-Communate;1235-39-8;1-Naphthalenecarboxylic acid, decahydro-1,4a-dimethyl-6-methylene-5-((2E)-3-methyl-2,4-pentadien-1-yl)-, methyl ester, (1S,4aR,5S,8aR)-;(12E)-Labda-8(17),12,14-triene-19-oic acid methyl ester;10178-35-5;(1S,8aalpha)-1,4abeta-Dimethyl-5beta-(3-methyl-2,4-pentadienyl)-6-methylenedecalin-1beta-carboxylic acid methyl e;methyl (1S,4aR,5S,8aR)-1,4a-dimethyl-6-methylidene-5-[(2E)-3-methylpenta-2,4-dienyl]-3,4,5,7,8,8a-hexahydro-2H-naphthalene-1-carboxylate;1-Naphthalenecarboxylic acid, decahydro-1,4a-dimethyl-6-methylene-5-(3-methyl-2,4-pentadienyl)-, methyl ester, [1S-[1.alpha.,4a.alpha.,5.alpha.(Z),8a.beta.]]-;Labda-8(20),12,14-trien-19-oic acid methyl ester;Labda-8(20),12,14-trien-19-oic acid, methyl ester;1-Naphthalenecarboxylic acid, decahydro-1,4a-dimethyl-6-methylene-5-(3-methyl-2,4-pentadienyl)-, methyl ester, [1S-(1.alpha.,4a.alpha.,5.alpha.,8a.beta.)]-;1-Naphthalenecarboxylic acid, decahydro-1,4a-dimethyl-6-methylene-5-(3-methyl-2,4-pentadienyl)-, methyl ester, [1S-[1.alpha.,4a.alpha.,5.alpha.(E),8a.beta.]]-