10.1139/V08-072
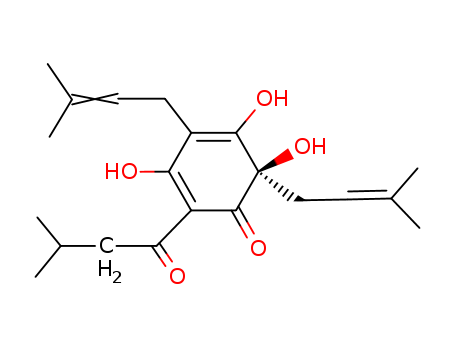
The study focuses on the photochemistry of trans-isohumulone, a key bitter flavoring component in beer, to understand its degradation under UV light, which is crucial for the brewing industry. The researchers irradiated methanolic solutions of trans-isohumulone with UV light at 313 nm, yielding four primary products containing an enolized cyclic β-triketone moiety: cis-isohumulone, humulone, dehydro-isohumulone, and dehydro-humulinic acid. Nine volatile products derived from the side chain of trans-isohumulone were also identified and quantified. The study aimed to clarify previous contradictory reports on the photolysis products of isohumulone and to provide insights into the unexpected photochemistry of alkenyl-substituted enolized cyclic β-triketones. The chemicals used in the study served as solvents, reagents for the irradiation process, and standards for the identification and quantification of the photoproducts.