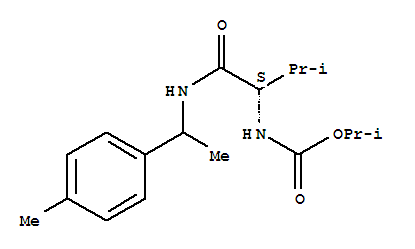
- Chemical Name:IPROVALICARB
- CAS No.:140923-17-7
- Molecular Formula:C18H28 N2 O3
- Molecular Weight:320.432
- Hs Code.:2930200012
- DSSTox Substance ID:DTXSID60861356
- Wikidata:Q27156987
- Mol file:140923-17-7.mol
Synonyms:Carbamicacid, [(1S)-2-methyl-1-[[[1-(4-methylphenyl)ethyl]amino]carbonyl]propyl]-,1-methylethyl ester (9CI); Fencaramid; Iprovalicarb; SZX 0722