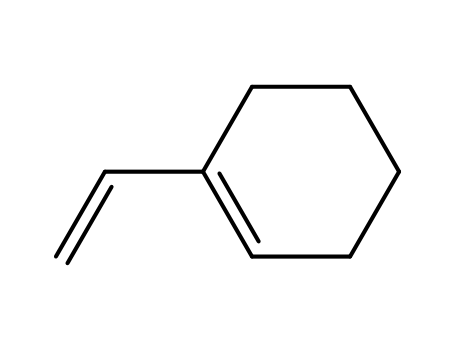
- Chemical Name:Vinylcyclohexene
- CAS No.:2622-21-1
- Molecular Formula:C8H12
- Molecular Weight:108.183
- Hs Code.:2902199090
- European Community (EC) Number:220-070-8
- DSSTox Substance ID:DTXSID6051934
- Nikkaji Number:J192.129K
- Wikidata:Q81984873
- Mol file:2622-21-1.mol
Synonyms:Vinylcyclohexene;1-Vinylcyclohexene;1-ETHENYLCYCLOHEXENE;Cyclohexene, 1-ethenyl-;Cyclohexene, ethenyl-;1-Vinyl-1-cyclohexene;Cyclohexene, 1-vinyl-;2-Vinylcyclohexene;2622-21-1;HSDB 5888;1-vinyl-cyclohexene;25168-07-4;EINECS 220-070-8;BRN 1236528;vinyl cyclohexene;1-vinylcyclohex-1-ene;1-ethenylcyclohex-1-ene;DTXSID6051934;AKOS006283559;LS-57552