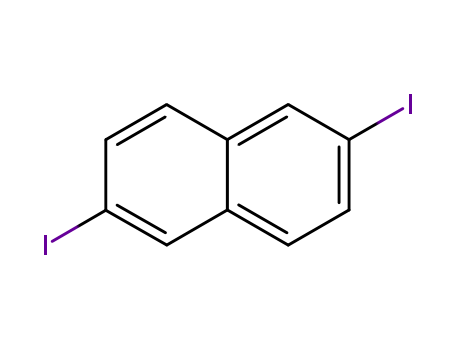
- Chemical Name:2,6-Diiodonaphthalene
- CAS No.:36316-88-8
- Molecular Formula:C10H6I2
- Molecular Weight:379.967
- Hs Code.:
- DSSTox Substance ID:DTXSID70189851
- Nikkaji Number:J1.618.406C
- Wikidata:Q83062062
- Mol file:36316-88-8.mol
Synonyms:2,6-diiodonaphthalene;36316-88-8;SCHEMBL1297632;DTXSID70189851;JEVDBSPYZIVTGM-UHFFFAOYSA-N;CS-0085713;FT-0725945;E84286