10.1021/jo00139a027
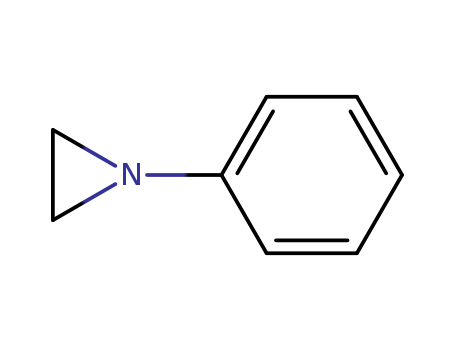
The research explores various aspects of organic chemistry, focusing on the reactions of hemiacetal esters with acids and alcohols, the synthesis and configuration determination of (R)-(-)-1,1,2-triphenyl-3,3-dimethylbutane, and the nitrogen-15 NMR and photoelectron spectroscopy of substituted N-phenylaziridines. The first study investigates the formation of mixed acetals and the thermolysis of hemiacetal esters using NMR spectroscopy, aiming to understand reaction mechanisms and equilibrium states. The second study synthesizes (R)-(-)-1,1,2-triphenyl-3,3-dimethylbutane and examines the stereochemistry of reactions involving benzhydryllithium and α-phenylneopentyl chloride, concluding that the optical purity of starting materials significantly affects the final product's configuration. The third study measures the 15N chemical shifts of N-arylaziridines and correlates them with shifts in anilines and anisoles, revealing high resonance dependence and smaller-than-expected steric effects. Key chemicals used across these studies include hemiacetal ester, acetic acid, diphenylmethyllithium, α-phenylneopentyl chloride, thionyl chloride, phosgene, and various substituted N-phenylaziridines.