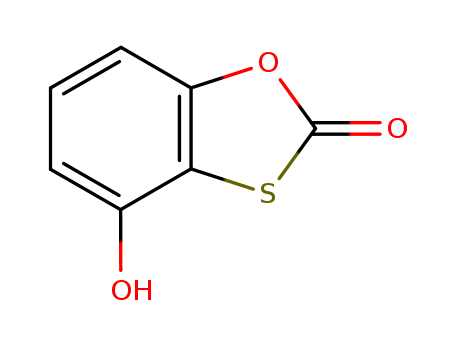
- Chemical Name:4-Hydroxy-1,3-(2H)-benzoxathiol-2-one
- CAS No.:95-18-1
- Molecular Formula:C7H4O3S
- Molecular Weight:168.173
- Hs Code.:
- NSC Number:71282
- UNII:37I4BS493F
- DSSTox Substance ID:DTXSID00241714
- Nikkaji Number:J39.067D
- Wikidata:Q27256683
- Mol file:95-18-1.mol
Synonyms:4-Hydroxy-1,3-(2H)-benzoxathiol-2-one;4-hydroxy-1,3-benzoxathiol-2-one;95-18-1;NSC-71282;1,3-Benzoxathiol-2-one, 4-hydroxy-;UNII-37I4BS493F;37I4BS493F;4-Hydroxy-1,3-benzoxathiol-2-one [INCI];NCIOpen2_000332;WLN: T56 BOVSJ FQ;SCHEMBL1962743;Resorcinol, cyclic thiocarbonate;DTXSID00241714;Resorcinol, cyclic 0,S-carbonate;NSC71282;Thiolcarbonic acid 4-hydroxy-o-phenylene ester;Q27256683;Carbonic acid, cyclic 0,S-ester with 2-mercaptoresorcinol;Carbonic acid, cyclic 0(1),s(2)-3-hydroxy-0-phenylene ester