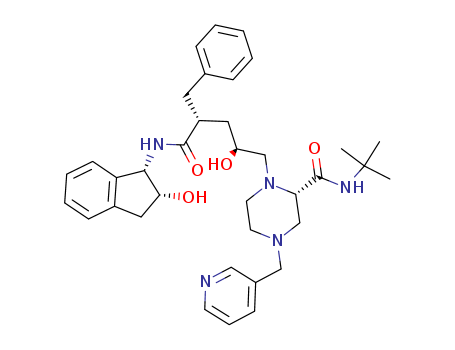
- Chemical Name:Indinavir
- CAS No.:150378-17-9
- Deprecated CAS:166746-42-5,216884-06-9
- Molecular Formula:C36H47N5O4
- Molecular Weight:613.8
- Hs Code.:
- UNII:9MG78X43ZT
- DSSTox Substance ID:DTXSID4043802
- Nikkaji Number:J566.361J
- Wikipedia:Indinavir
- Wikidata:Q425490
- NCI Thesaurus Code:C74585
- Pharos Ligand ID:3WRTT7LPSHQR
- Metabolomics Workbench ID:42626
- ChEMBL ID:CHEMBL115
- Mol file:150378-17-9.mol
Synonyms:Crixivan;Indinavir;Indinavir Sulfate;Indinavir, Sulfate (1:1);L 735 524;L 735,524;L-735 524;L-735,524;L735 524;L735,524;MK 639;MK-639;MK639;Sulfate, Indinavir