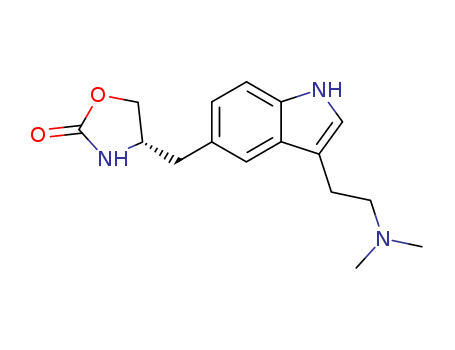
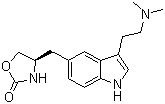
- Chemical Name:Zolmitriptan
- CAS No.:139264-17-8
- Molecular Formula:C16H21N3O2
- Molecular Weight:287.362
- Hs Code.:29349990
- European Community (EC) Number:629-919-0
- NSC Number:760383
- UNII:2FS66TH3YW
- DSSTox Substance ID:DTXSID8045933
- Nikkaji Number:J694.097H
- Wikipedia:Zolmitriptan
- Wikidata:Q218820
- NCI Thesaurus Code:C47789
- RXCUI:135775
- Pharos Ligand ID:ADTFHLBU6LVP
- Metabolomics Workbench ID:42701
- ChEMBL ID:CHEMBL1185
- Mol file:139264-17-8.mol
Synonyms:311C90;4-((3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)methyl)-2-oxazolidinone;AscoTop;Flezol;zolmitriptan;Zomig;Zomigoro


 Xi
Xi

