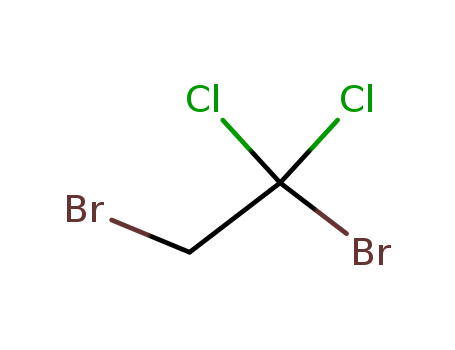
- Chemical Name:1,2-Dibromo-1,1-dichloroethane
- CAS No.:75-81-0
- Molecular Formula:C2H2 Br2 Cl2
- Molecular Weight:256.752
- Hs Code.:
- European Community (EC) Number:200-904-7
- NSC Number:6199
- UNII:8D6FN8F1WD
- DSSTox Substance ID:DTXSID8058790
- Nikkaji Number:J351D
- Wikidata:Q27270205
- Mol file:75-81-0.mol
Synonyms:1,2-DIBROMO-1,1-DICHLOROETHANE;75-81-0;Ethane, 1,2-dibromo-1,1-dichloro-;1,2-Dibromo-2,2-dichloroethane;HSDB 2777;NSC 6199;EINECS 200-904-7;UNII-8D6FN8F1WD;BRN 1697498;8D6FN8F1WD;AI3-14678;NSC-6199;1,2-Dibromo-1,1-dichloroethane 100 microg/mL in Methanol;Ethane, dibromodichloro-;NSC6199;C2H2Br2Cl2;SCHEMBL728264;1,2-dibrom-1,1-dichlorethan;DTXSID8058790;Ethane,2-dibromo-1,1-dichloro-;1,2-dibromo-1,1-dichloro-ethane;MFCD00053228;AKOS025310201;1,1-DICHLORO-1,2-DIBROMOETHANE;1,2-Dibromo-1,1-dichloroethane, 97%;LS-65479;EN300-344060;1,2-DIBROMO-2,2-DICHLOROETHANE [HSDB];Q27270205