10.1002/chem.201804416
The study, authored by Nicola Lucchetti and Ryan Gilmour, presents a novel approach to chemical glycosylation, which is a critical process in the synthesis of carbohydrates. It introduces a direct, metal-free method for the anomeric O-arylation of unactivated carbohydrates, bypassing the need for pre-functionalization of the substrate. This method employs stable aryliodonium salts to facilitate a formal O?H functionalization reaction at ambient temperature, leading to the formation of acetals directly from reducing sugars. The research demonstrates the process's efficiency and stereoretention with various monosaccharides, disaccharides, and trisaccharides, including those with fluorinated substrates to enhance the anomeric effect. The method's scalability is also validated, and it is suggested that this strategy could be broadly applicable for constructing complex acetals in carbohydrate chemistry.
10.1016/j.bmcl.2004.02.092
The study focuses on the design, synthesis, and evaluation of a novel class of pyrazolo[3,4-d]pyrimidines as potent inhibitors of enteroviruses, specifically coxsackieviruses. The researchers synthesized a series of these compounds and tested their antiviral activity using a plaque reduction assay. They discovered that these compounds showed remarkable specificity for human enteroviruses, with some derivatives highly effective at nanomolar concentrations. Structure-activity relationship (SAR) studies indicated that the phenyl group at the N-1 position and the hydrophobic diarylmethyl group at the piperazine significantly influenced the in vitro antienteroviral activity. Notably, compounds with a thiophene substituent, such as 20–24, exhibited high activity against coxsackievirus B3 and moderate activity against enterovirus 71, without apparent cytotoxic effects on RD cell lines. The findings highlight the potential of these compounds as new antiviral agents against enteroviral infections, for which effective treatments are currently lacking.
10.1055/s-0035-1560565
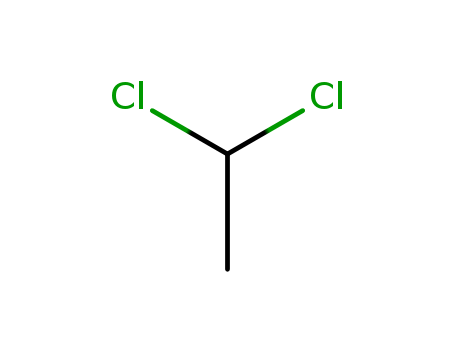
The research focuses on the type 2 ring-opening reactions of cyclopropanated 7-oxabenzonorbornadienes with carboxylic acid nucleophiles. The experiments were designed to investigate the efficiency of these reactions under various conditions, using p-toluenesulfonic acid monohydrate as a catalyst in dichloroethane at 90°C. The study explored the impact of different acid catalysts, reaction temperatures, solvents, and nucleophile equivalencies on the reaction yield and rate. A range of carboxylic acids with varying pKa values were tested as nucleophiles, and the reactions were analyzed using techniques such as NMR, IR spectroscopy, and HRMS to characterize the products, which were 2-naphthylmethyl esters. The results showed a dependence of reaction rates on the acidity of the nucleophiles, with the optimal conditions yielding esters in moderate to decent yields. The study provides a new route for the synthesis of 2-naphthylmethyl esters and insights into the reactivity of similar fused-ring systems.
10.1021/co300046r
The research focuses on the development of a one-pot, two-step protocol for synthesizing a variety of novel polycyclic heterocycles with high skeletal diversity, characterized by the presence of embedded or attached benzimidazole and often a ring system formed through lactamization. The study utilizes the Ugi multicomponent reaction (MCR) combined with an acid-mediated cyclodehydration step. Reactants include keto- or formyl acids, N-Boc-diamines, and isocyanides, which are combined in trifluoroethanol (TFE) as the solvent. The Ugi reaction is monitored using LCMS, and after completion, the product is subjected to a treatment with trifluoroacetic acid (TFA) in dichloroethane (DCE) and microwave irradiation to promote cyclodehydration, resulting in the formation of tricyclic scaffolds with an α-quaternary methyl group. The experiments yield a range of unique scaffolds with excellent physicochemical properties, demonstrating the versatility and efficiency of the protocol. The analysis of the products includes purification using a CombiFlash Rf system and X-ray crystallographic analysis for structural elucidation, confirming the successful synthesis of the desired polycyclic heterocycles.
10.1039/c3cc46361c
The research focuses on the development of a novel heteroannulation reaction between benzynes and N-aryl α-aminoketones, leading to the synthesis of N-aryl-2,3-disubstituted indoles. This method represents a significant advancement as it enables, for the first time, a one-step synthesis of unsymmetrical 2,3-dialkyl (non-methyl) substituted indoles with complete control over regioselectivity. The chemicals used in this process include 2-(trimethylsilyl)aryl triflates (such as 1a), N-aryl-α-aminoketones (such as 2a), CsF as a fluoride source, Cs2CO3 as a base, and 18-C-6 as a crown ether additive. The optimized reaction conditions involved using a solvent mixture of isopropionitrile/dichloroethane, carried out at room temperature, which effectively suppressed the formation of side products and improved the overall yield of the desired indole products. The study concluded that this new method has a wide application scope and offers a valuable solution to a longstanding challenge in the field of indole synthesis.
10.1039/c5cc07598j
The research focuses on the development of an efficient copper-catalyzed intramolecular transannulation reaction of pyridotriazoles with internal alkynes, leading to the synthesis of various fused polycyclic indolizines. This method provides a general access to tri-, tetra-, and pentacyclic fused indolizines, including C6-substituted fused indolizines, which are challenging to synthesize selectively through known methods. The study also reveals, for the first time, that this reaction can proceed via a Lewis acid-mediated electrophilic pathway, in addition to the established Rh- or Cu-catalyzed carbene mechanism. The experiments involved an extensive screening of transition metal catalysts and optimized conditions using CuBr-SMe2 as the catalyst in dichloroethane at 140°C. The scope of the reaction was explored with various substrates, including aryl, alkenyl, and alkyl alkynes, as well as electron-deficient alkynes, demonstrating the reaction's generality and compatibility with different functional groups. The reaction products were analyzed and confirmed through standard analytical techniques, and the yields of the products were reported.


 Xi
Xi F
F Xn
Xn T
T


