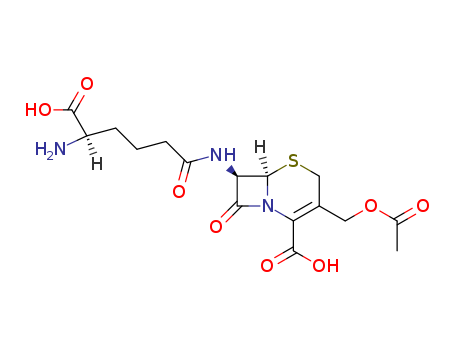
- Chemical Name:cephalosporin C
- CAS No.:61-24-5
- Molecular Formula:C16H21 N3 O8 S
- Molecular Weight:415.424
- Hs Code.:
- European Community (EC) Number:200-501-6,254-669-0
- UNII:3XIY7HJT5L
- DSSTox Substance ID:DTXSID90960427
- Nikkaji Number:J4.815A
- Wikipedia:Cephalosporin_C
- Wikidata:Q5063335
- Metabolomics Workbench ID:50256
- ChEMBL ID:CHEMBL482858
- Mol file:61-24-5.mol
Synonyms:cephalosporin C;cephalosporin C hydrochloride;cephalosporin C, monosodium salt;cephalosporin C, monosodium salt, (6R-(6alpha,7beta))-isomer;cephalosporin C, monozinc salt;cephalosporin C, potassium salt;cephalosporin C, potassium salt, (6R-(6alpha,7beta(S*)))-isomer;cephalosporin C, sodium salt;cephalosporin C, sodium salt, (6R-(6alpha,7beta))-isomer;cephalosporin C, zinc salt