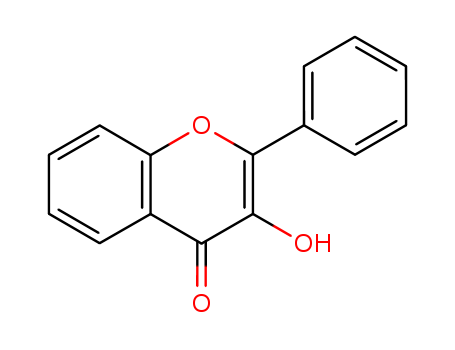
- Chemical Name:3-Hydroxyflavone
- CAS No.:577-85-5
- Molecular Formula:C15H10 O3
- Molecular Weight:238.243
- Hs Code.:2932999099
- European Community (EC) Number:209-416-9
- NSC Number:57653
- UNII:ZTG9LSS5QH
- DSSTox Substance ID:DTXSID4060365
- Nikkaji Number:J1.628D,J630.345E
- Wikipedia:3-Hydroxyflavone
- Wikidata:Q5919049
- NCI Thesaurus Code:C68456
- Pharos Ligand ID:1QZLHPK5TMK5
- Metabolomics Workbench ID:45126
- ChEMBL ID:CHEMBL294009
- Mol file:577-85-5.mol
Synonyms:3-hydroxy-2-phenyl-4H-1-benzopyran-4-one;3-hydroxy-2-phenylchromone;3-hydroxyflavone;flavonol


 Xi
Xi

