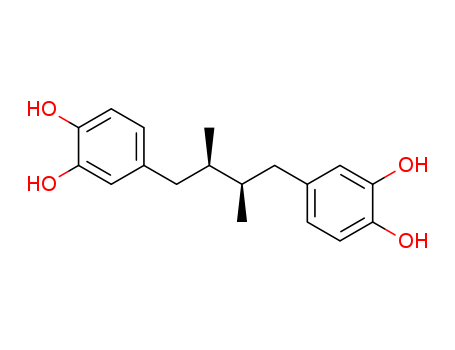
- Chemical Name:Masoprocol
- CAS No.:27686-84-6
- Deprecated CAS:334707-72-1,741285-10-9,1050512-02-1,741285-10-9
- Molecular Formula:C18H22O4
- Molecular Weight:302.37
- Hs Code.:
- European Community (EC) Number:248-606-6
- UNII:7BO8G1BYQU
- DSSTox Substance ID:DTXSID5045178
- Nikkaji Number:J257.424A
- Wikipedia:Masoprocol
- Wikidata:Q6783851
- NCI Thesaurus Code:C701
- RXCUI:227239
- Pharos Ligand ID:NPKM56RHNKN7
- Metabolomics Workbench ID:42587
- ChEMBL ID:CHEMBL313972
- Mol file:27686-84-6.mol
Synonyms:1,2-Benzenediol,4,4'-(2,3-dimethyl-1,4-butanediyl)bis-, (R*,S*)- (9CI);Pyrocatechol, 4,4'-(2,3-dimethyltetramethylene)di-,meso- (8CI);Actinex;CHX 100;Masoprocol;meso-NDGA;meso-Nordihydroguaiareticacid;4-[(2S,3R)-4-(3,4-dihydroxyphenyl)-2,3-dimethylbutyl]benzene-1,2-diol;1,2-Benzenediol, 4,4'-(2,3-dimethyl-1,4-butanediyl)bis-;4,4'-(2,3-Dimethyl-1,4-butanediyl)bis[1,2-benzenediol];4,4'-(2,3-Dimethylbutane-1,4-diyl)dibenzene-1,2-diol;