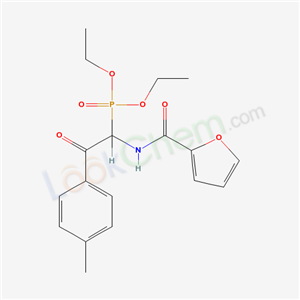
- Chemical Name:1,2-Dichloro-3-nitronaphthalene
- CAS No.:6240-55-7
- Molecular Formula:C10H5Cl2NO2
- Molecular Weight:242.06
- Hs Code.:
- DSSTox Substance ID:DTXSID90211466
- Nikkaji Number:J34.571G
- Wikidata:Q83086391
- Mol file:6240-55-7.mol
Synonyms:1,2-Dichloro-3-nitronaphthalene;6240-55-7;BRN 2118398;NAPHTHALENE, 1,2-DICHLORO-3-NITRO-;AI3-26497;SCHEMBL7827176;DTXSID90211466;LS-94552