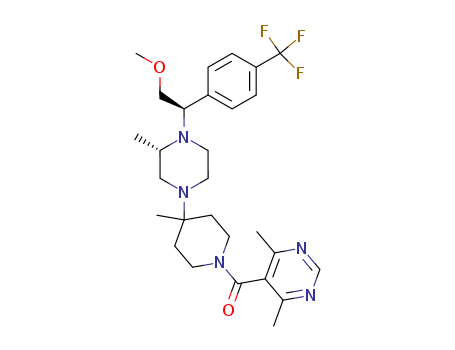
- Chemical Name:Vicriviroc
- CAS No.:306296-47-9
- Molecular Formula:C28H38F3N5O2
- Molecular Weight:533.637
- Hs Code.:
- UNII:TL515DW4QS
- DSSTox Substance ID:DTXSID40897719
- Nikkaji Number:J1.905.357A
- Wikipedia:Vicriviroc
- Wikidata:Q3557001
- NCI Thesaurus Code:C73589
- Pharos Ligand ID:9NCBGUWKMNJH
- Metabolomics Workbench ID:149882
- ChEMBL ID:CHEMBL82301
- Mol file:306296-47-9.mol
Synonyms:1-((4,6-dimethyl-5-pyrimidinyl)carbonyl)-4-(4-(2-methoxy-4-(trifluoromethyl)phenyl)ethyl-3-methyl-1-piperazinyl)-4-methylpiperidine;Sch 417690;Sch-417690;Sch417690;vicriviroc