Base Information
Edit
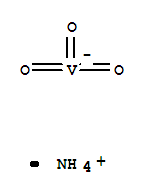
- Chemical Name:Ammonium vanadate(V)
- CAS No.:7803-55-6
- Molecular Formula:H4N.O3V
- Molecular Weight:116.98
- Hs Code.: Oral rat LD50: 58100 ug/kg
- Mol file:7803-55-6.mol
Synonyms:Ammoniumvanadate(V) ((NH4)VO3) (6Cl,7Cl);CCRIS 4120;Vanadicacid (HVO3), ammonium salt (8Cl);HSDB 6310;Ammoniummetavanadate;Ammonium metavanadate (NH4VO3);Ammoniumtrioxovanadate;Ammonium trioxovanadate(1-);Ammonium vanadate;Ammoniumvanadate ((NH4)VO3);Ammonium vanadium oxide (NH4VO3);Ammonium vanadium trioxide;NSC 215196;


 T+,
T+, T
T
 T+:Very toxic;
T+:Very toxic;