- Chemical Name:Amantadine
- CAS No.:768-94-5
- Deprecated CAS:744952-70-3
- Molecular Formula:C10H17N
- Molecular Weight:151.252
- Hs Code.:29213000
- European Community (EC) Number:212-201-2
- NSC Number:341865
- UNII:BF4C9Z1J53
- DSSTox Substance ID:DTXSID8022117
- Nikkaji Number:J6.971J
- Wikipedia:Amantadine
- Wikidata:Q409761,Q27453436
- NCI Thesaurus Code:C61632
- RXCUI:620
- Pharos Ligand ID:CB68GN711F4T
- Metabolomics Workbench ID:43183
- ChEMBL ID:CHEMBL660
- Mol file:768-94-5.mol
Synonyms:1 Aminoadamantane;1-Aminoadamantane;Adamantylamine;Adekin;AL, Amantadin;Aman;Amanta;Amanta HCI AZU;Amanta Sulfate AZU;Amanta-HCI-AZU;Amanta-Sulfate-AZU;Amantadin AL;Amantadin AZU;Amantadin neuraxpharm;Amantadin ratiopharm;Amantadin Stada;Amantadin-neuraxpharm;Amantadin-ratiopharm;Amantadina Juventus;Amantadina Llorente;Amantadine;Amantadine Hydrochloride;Amantadine Sulfate;Amantadinneuraxpharm;Amantadinratiopharm;AmantaHCIAZU;AmantaSulfateAZU;Amixx;AZU, Amantadin;Cerebramed;Endantadine;Gen Amantadine;Gen-Amantadine;GenAmantadine;Hydrochloride, Amantadine;Infecto Flu;Infecto-Flu;InfectoFlu;Infex;Juventus, Amantadina;Llorente, Amantadina;Mantadix;Midantan;PMS Amantadine;PMS-Amantadine;PMSAmantadine;Stada, Amantadin;Sulfate, Amantadine;Symadine;Symmetrel;tregor;Viregyt;Wiregyt

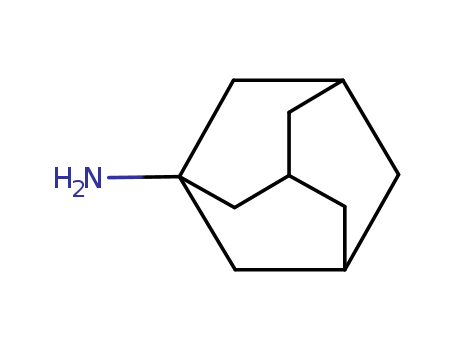

 Xn,
Xn,  Xi
Xi


