10.1021/jm00279a031
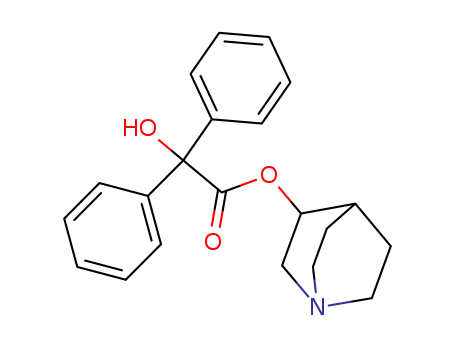
The research encompasses three distinct studies focusing on the synthesis and evaluation of various chemical compounds for their pharmacological properties. The first study investigates the specific rotation, melting points, and pharmacological effects of different isomers of 3-quinuclidinyl benzilate, concluding that the levorotatory isomer is significantly more potent than its dextrorotatory counterpart. The second study explores the anabolic and androgenic activities of esters of 19-nortestosterone with acyclic and cyclic terpenyl acids in rats, identifying 19-nortestosterone homofarnesate as a promising anabolic agent with a long duration of action and minimal androgenic effects. The third study synthesizes and evaluates terpenyl carbamates for their central nervous system (CNS) depressant and anticonvulsant activities, with compound 1 showing significant CNS depression and anticonvulsant effects but lacking central analgesic activity.


 Xn
Xn


