10.1021/ol0703940
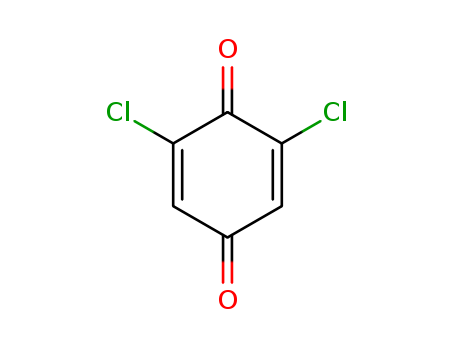
The research focuses on the cross-metathesis reaction between r-methylene-γ-butyrolactone and terminal olefins, aiming to develop an efficient and highly stereoselective method for synthesizing r-alkylidene-γ-butyrolactones, which are valuable building blocks in organic synthesis. The experiments involved using various ruthenium-based catalysts, with [Ru]-II being the most effective, and a range of additives to enhance the reaction efficiency and minimize the formation of undesired isomerized byproducts. The reactions were conducted in refluxing CH2Cl2, with the products and reaction progress analyzed using 1H NMR spectroscopy. Key additives identified were chlorocatecholborane and 2,6-dichloro-1,4-benzoquinone, which significantly improved the reaction's yield and selectivity. The study also explored the reaction with a variety of olefinic partners, achieving moderate to excellent yields with high E-stereoselectivity.